Article By ELIZABETH HOFHEINZ, M.P.H., M.ED. • WED, JANUARY 25TH, 2023
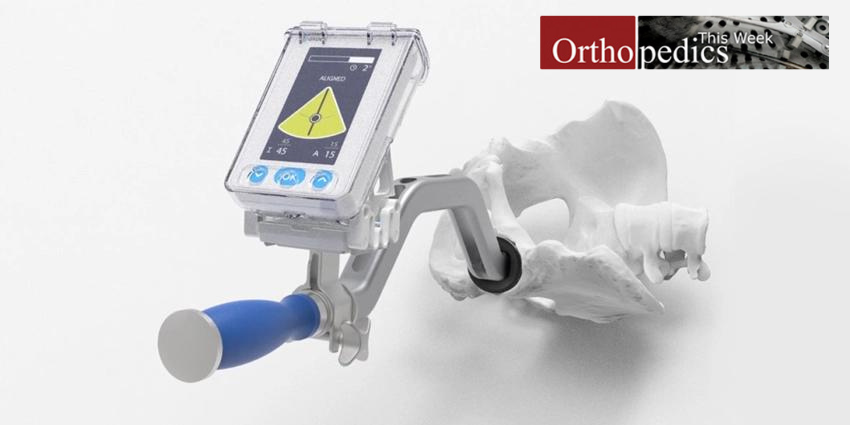
A truly novel, pinless navigation system designed to improve the accuracy of positioning the acetabular cup in the pelvis during hip replacement procedures is in the process of entering the U.S. market.
Among the noteworthy aspects of both this navigation system and the Australian company that developed and is commercializing it, is that its core DNA is derived from modern aeronautical navigation. Additionally, the company, named Gyder Surgical, is led by an extraordinary team with roots in Synthes and Johnson & Johnson.

Gyder Surgical’s CEO, Sujit Dike
Gyder’s non-invasive Pin-less GYDER™ Hip Navigation System was featured in early January 2023 at the premier high technology showcase meeting—the Biotech Showcase™ 2023 Digital Medicine & Medtech conference held in Palo Alto, California—the heart of Silicon Valley.
Presenting on behalf of Gyder was its CEO, Sujit Dike—a veteran Johnson & Johnson executive, former Roam Robotics exec and member, along with long time orthopedic executive Nick Pachuda, of Gyder Surgical’s board of directors.
Dike and OTW held extensive discussions about Gyder’s innovative approach to large joint reconstruction navigation.
Dike is convinced that Gyder’s patented pinless technology—a noninvasive approach—has the potential to vastly improve the surgeon and patient experience. “For one thing,” explained Dike to OTW, “it is faster because there is no need to insert pins into the pelvis and no need to use the pins for registration landmarks.”
“Gyder’s technology also does not require pre-operative or intraoperative imaging. If you look at the overall setup, calibration takes one minute, and registration can be done in one step.”
“I am elated at the opportunity to bring this Australian technology to the U.S. and other markets around the world,” says Dike. “We anticipate that the GYDER Hip Navigation System will be available in the U.S. in 2023, pending regulatory approval.”
When Wharton educated and U.S. based Sujit Dike hit Australia’s shores to join Gyder, he was prepared to tackle a ubiquitous problem in medicine—the pressure on surgeons, hospitals, and ambulatory surgery centers (ASCs), to improve outcomes, increase patient satisfaction while doing more cases at greater cost efficiencies.
Dike joined Gyder in May 2022 as its new CEO and member of the board of directors. Dike brings to his new role 20+ years industry experience in orthopedics, general surgery, imaging, navigation and genomics.
“I was thrilled to lead Gyder, a company that really understands intuitive, non-invasive, smart navigation technology for orthopedic applications,” said Dike.
Dike began his career in orthopedic spine and trauma at West Chester, Pennsylvania-based Synthes, Inc. Following Johnson & Johnson’s 2012 purchase of Synthes, Dike remained with the company and was assigned several commercial and strategic leadership roles at J&J. One was vice president of the J&J MedTech ASC Channel and Enabling Technologies group, where he was responsible for developing J&J’s ASC channel operating strategies and led a ASC-focused commercial team across orthopedics and general surgery.
One particularly unique career experience, says Dike, was working for innovative small companies like Affymetrix, a gene sequencing pioneer. “It was exciting to work for the first company to commercialize gene sequencing.”
“I also worked for a startup called Roam Robotics, a visionary company making breakthrough wearable robotics/exoskeleton technology. Both of these experiences taught me to focus on developing organizations that can bring innovation at a rapid speed and efficient scale to market. In addition, I learned to contend with the inevitable complexity involved in being a small company, i.e., how to be resource efficient and maintain a focus on customers.”
Sujit Dike earned an M.B.A. from the Wharton School of the University of Pennsylvania, a Masters in Biochemistry and Molecular Biology from SUNY Stony Brook in New York, and an undergraduate degree in Biotechnology & Biochemical Engineering from the Indian Institute of Technology, Delhi.
He is the lead and co-author of more than nine peer-reviewed publications in leading scientific journals including Nature Genetics, Genome Research and Science, and is a recipient of the U.S. Dept of Agriculture award for significant contributions to the global rice genome sequencing initiative.
OTW asked Dike about the challenges of bringing truly novel innovations to the notoriously conservative orthopedic surgeon market, he offered an interesting and practical perspective. “Business leaders underestimate the importance of prioritization and focusing on what is most important to customers.”
Speaking of other high-level challenges, Dike continued, “Reimbursement and regulatory pathways for innovative technologies are constantly evolving, and these pose challenges when bringing products into key markets.”
“To ensure that you have the support of regulatory agencies and payors, developing evidence-based data and early engagement with agencies is key. Solving a major problem is never easy for a small startup company with limited resources, but when you are laser focused like Gyder you become very efficient at delivery, nimbleness, flexibility, and you hone the ability respond to changing dynamics.”
“Surgeons are under more pressure than ever,” said Dike, “but they haven’t had the right tools to handle these challenges. Most tools are complex and slow and add procedure time and expense, particularly in hip arthroplasty. I was immediately fascinated with Gyder because here was a hip arthroplasty navigation technology that has the right combination of fast and easy. It’s simple to use, requires minimal training, and is cost effective in improving acetabular cup placement.”
“In recent years,” commented Dike to OTW, “physicians in Australia have shown strong interest in incorporating new technology in their practice, recognizing the value it brings in delivering better patient care. A relatively favorable regulatory environment has enabled companies to increasingly view Australia as a target market for early commercialization. Similar to the United States, insurance providers are increasingly supporting the growing trend of performing joint replacements in orthopedic outpatient centers. The Gyder technology, being an Australian invented, developed and manufactured product, is well placed to capitalize on these trends.”
Gyder, under Dike’s leadership, will, we think, evolve to become one of the most consequential young digital technology companies in Orthopedics. Stay tuned, for sure.